previous year questions chemical reactions and equations class 10:CBSE

Class 10 chemistry important questions with answers are provided here for Chapter 1 Chemical Reactions and Equations. These important questions are based on CBSE board curriculum and correspond to the most recent Class 10 chemistry syllabus. By practising these Class 10 important questions, students will be able to quickly review all of the ideas covered in the chapter and prepare for the Class 10 Annual examination.
We have given these Important Questions for Class 10 Science Chapter 1 Chemical Reactions and Equations to solve different types of questions in the exam. Previous Year Questions & Important Questions of Chemical Reactions and Equations Class 10 Science Chapter 1 will help the students to score good marks in the board examination.
Download Class 10 Chemistry Chapter 1 Chemical Reactions and Equations Important Questions with Answers PDF by clicking on the button below
Chemical Reaction and Equation
Previous Years’ CBSE Board Questions
1.1 Chemical Equations
Q.1. Sodium reacts with water to form sodium hydroxide and hydrogen gas. Thebalanced equation which represents the given reaction is . 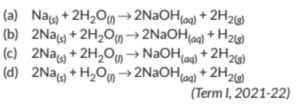
Ans.:- (b)
Q.2. It is important to balance the chemical equations to satisfy the law ofconservation of mass. Which of the following statements of the law isincorrect?
(a) The total mass of the elements present in the reactants is equal to the total
mass of the elements present in the products.
(b) The number of atoms of each element remains the same, before and after a
chemical reaction.
(c) The chemical composition of the reactants is the same before and after the
reaction.
(d) Mass can neither be created nor can it be destroyed in a chemical reaction.
(Term 1, 2021-22)
ANS.:- (c)
Q3. In which of the following, the identity of initial substance remains
unchanged?
(a) Curdling of milk
(b) Formation of crystals by process of crystallisation
(c) Fermentation of grapes
(d) Digestion of food (2020)
Ans.:-(b)
4. Identify ‘x, ‘y’ and ‘z’ in the following reaction: 2KCIO32KC+026)
(a) x=gas: y = reaction condition; z = gas
(b) x=solid; y = liquid; z = gas
(c) x = number of moles of KCIO3: y = reaction condition; z = number of
molecules of oxygen
(d) x = physical state of KCIO, and KCI; y = reaction condition, z=physical
state of O₂ (2020)
Ans:-(C)
5. Assertion (A): Following is a balanced chemical equation for the action of
steam on iron: 3Fe+ 4H2O-Fe3O4+4H2
Reason (R): The law of conservation of mass holds good for a chemical
equation.
(a) Both (A) and (R) are true and reason (R) is the correct explanation of the
assertion (A).
(b) Both (A) and (R) are true, but reason (R) is not the correct explanation of
the assertion (A).
(c) (A) is true, but (R) is false.
(d) (A) is false, but (R) is true. (2020)
Ans.:-(c)
VSA (1 mark)
Q.6. What is a balanced chemical equation? (2021 C) Ans:-6. The equation which contains an equal number of atoms of each element on
both sides of the arrow is called a balanced chemical equation.
Q,7. Translate the following statement into a balanced chemical equation:
“Barium chloride reacts with aluminium sulphate to give aluminium chloride
and barium sulphate.” (2019) Ans:- 3BaCl2 + Al2(SO4)3 → 2AlCl3 + 3BaSO4
SAI (2 marks)
8. Give the chemical name of the reactants as well as the products of the
following chemical equation: HNO3 + Ca(OH)2 → Ca(NO3)2 + H2O (2021 C) Ans:-. Reactants:- Nitric acid, calcium hydroxide (slaked lime) Products:- Calcium
nitrate, water
SA II (3 marks)
Q.9. Explain the significance of photosynthesis. Write the balanced chemical
equation involved in the process. (Board Term I, 2017) U Ans.:-Photosynthesis means synthesis with the help of light. It is the process that
gives life to all living beings. Photosynthesis is a process by which plants utilize carbon dioxide and water in the presence of sunlight to produceglucose and oxygen. 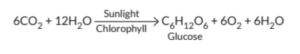
Q.10. Write balanced chemical equations for the following chemical reactions:
(a) Hydrogen+Chlorine→→→ Hydrogen chloride (NCERT Intext)
(b) Lead + Copper chloride →→ Lead chloride +Copper (Board Term 1, 2014)
(c) Zinc oxide + Carbon→ Zinc+Carbon monoxide Ans.:- (a) H2(g) + Cl2(g) → 2HCl (g)
(b) Pb (s) + CuCl2(aq.) → PbCl2(aq.) + Cu (s) (c) ZnO(s) + C (s) → Zn(s) + CO(g)
Chemical Reaction and Equation
Previous Years’:- Watch on you tube
Q.11. Assertion (A): Burning of natural gas is an endothermic process.
Reason (R): Methane gas combines with oxygen to produce carbon dioxide
and water.
(a) Both (A) and (R) are true and (R) is the correct explanation of (A).
(b) Both (A) and (R) are true but (R) is not the correct explanation of (A).
(c) (A) is true, but (R) is false.
(d) (A) is false, but (R) is true. (Term I, 2021-22) Ans.:-(d)
Q.12. Consider the following processes
I. Dilution of sulphuric acid
II. Sublimation of dry ice
III. Condensation of water vapours
IV. Dissolution of ammonium chloride in water
The endothermic process(es) is/are
(a) I and III
(b) II only
(c) Ill only
(d) II and IV (Term 1, 2021-22) Ans.:-(d)
Q.13. When lead nitrate powder is heated in boiling tube, we observe
(a) brown fumes of nitrogen dioxide
(b) brown fumes of lead oxide
(c) yellow fumes of nitrogen dioxide
(d) brown fumes of nitric oxide. (Term 1, 2021-22) Ans.:-(a)
Q.14. Assertion (A): Silver salts are used in black and white photography.
Reason (R): Silver salts do not decompose in the presence of light.
(a) Both (A) and (R) are true and (R) is the correct explanation of (A).
(b) Both (A) and (R) are true but (R) is not the correct explanation of (A).
(c) (A) is true, but (R) is false.
(d) (A) is false, but (R) is true. (Term I, 2021-22) Ans.:-(c)


Our e-pharmacy offers a wide range of health products with competitive pricing.
Shoppers will encounter both prescription and over-the-counter drugs suitable for different health conditions.
We strive to maintain trusted brands while saving you money.
Quick and dependable delivery provides that your medication gets to you quickly.
Experience the convenience of shopping online on our platform.
what is in kamagra